FLOWFLEX™ COVID-19 ANTIGEN HOME TEST – SINGLE USE
Qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection. This test is authorized for non-prescription home use with self-collected anterior nasal swab specimens directly from individuals aged 14 years and older or with adult-collected anterior nasal samples directly from individuals aged 2 years or older. The Flowflex COVID-19 Antigen Home Test does not require serial testing.
• Anterior nasal swab specimens
• Results in 15 minutes
• 12 Months shelf life
• Store between 36 to 86° F
• Sample self-collection ages 14 and older
• Sample collection by an adult in children ages 2 to 13
• Excellent performance when compared to an FDA
authorized molecular SARS-CoV-2 test.
$19.95
Description
A rapid test for the detection of SARS-CoV-2 antigens in anterior nasal specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection.
Clinical Performance
The Flowflex COVID-19 Antigen Home Test was compared to an FDA authorized molecular SARS-CoV-2 test. The Flowflex COVID-19 Antigen Home Test correctly identified 93% of positive specimens and 100% of negative specimens.
Materials Provided
• Test Cassette
• Package Insert
• Extraction Buffer Tube
• Nasal Swab
• External Tube Holder
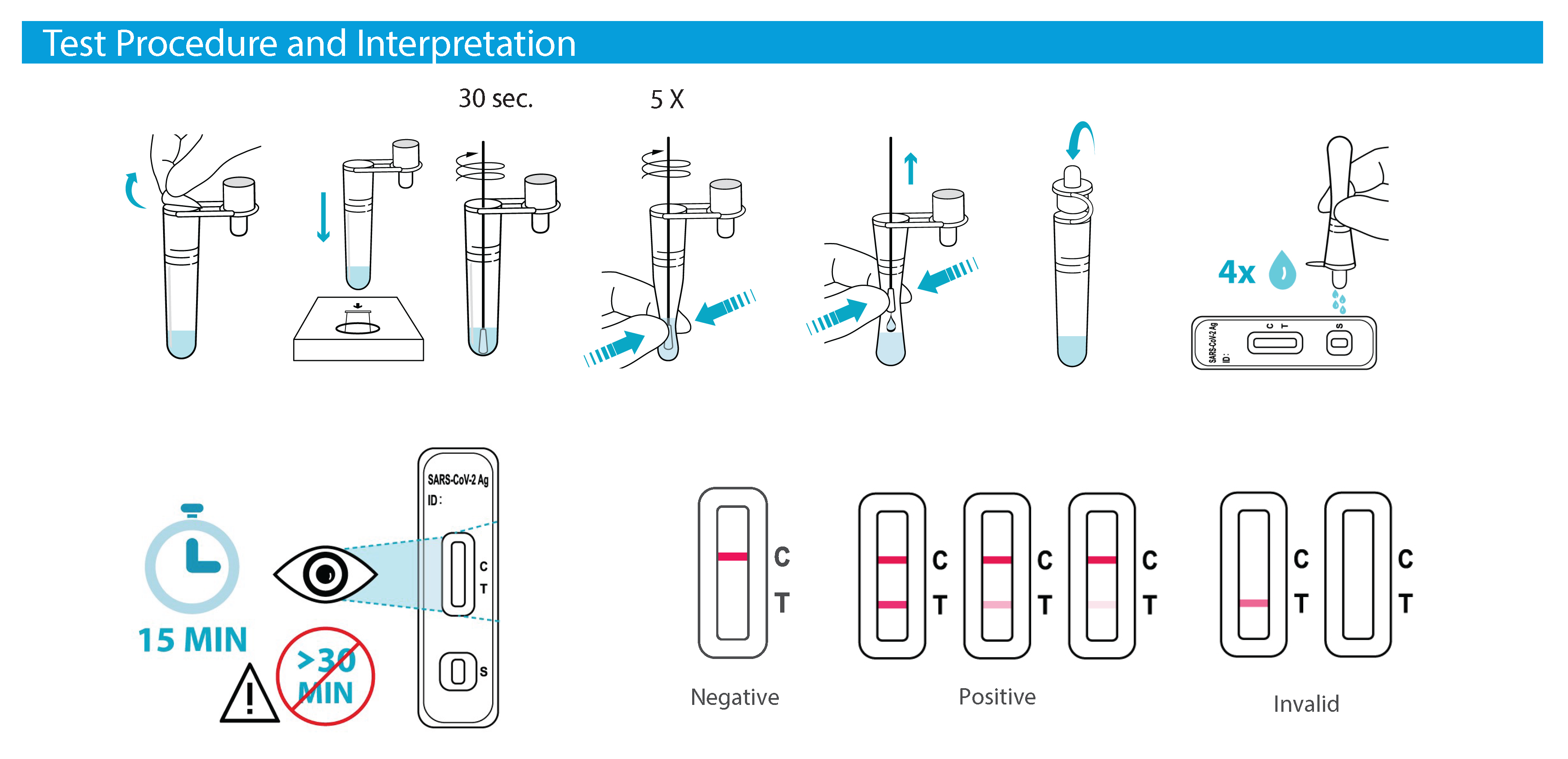
How deep should I insert the swab into my nose?
Insert the swab ½ to ¾ inches inside your nostril. With children, the maximum depth of insertion into the nostril may
be less than ¾ of an inch, and you may need to have a second person hold the child’s head while swabbing.
Note: A false-negative result may occur if the nasal swab specimen is not properly collected.
• This product has not been FDA cleared or approved but has been authorized by FDA under an EUA.
• This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
• The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVDs for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
• For more information on EUAs please visit: https://www.fda.gov/emergency-preparednessand-response/mcm-legal-regulatory-and-policy-framework/emergency-useauthorization
• For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19
• For detailed instructions, please visit: www.aconlabs.com
Related products
-
- Compression Stockings, Health and Wellness Medical Supplies
COMPRESSION STOCKINGS SOFT-MICRO™
- $30.00 – $66.00
- Select options This product has multiple variants. The options may be chosen on the product page
-
- Compression Stockings, Health and Wellness Medical Supplies
COMPRESSION STOCKINGS SHEER VIBRANCE™
- $14.00 – $90.00
- Select options This product has multiple variants. The options may be chosen on the product page
-
- Compression Stockings, Health and Wellness Medical Supplies
COMPRESSION STOCKINGS ACTIVEWEAR™
- $30.00 – $40.00
- Select options This product has multiple variants. The options may be chosen on the product page